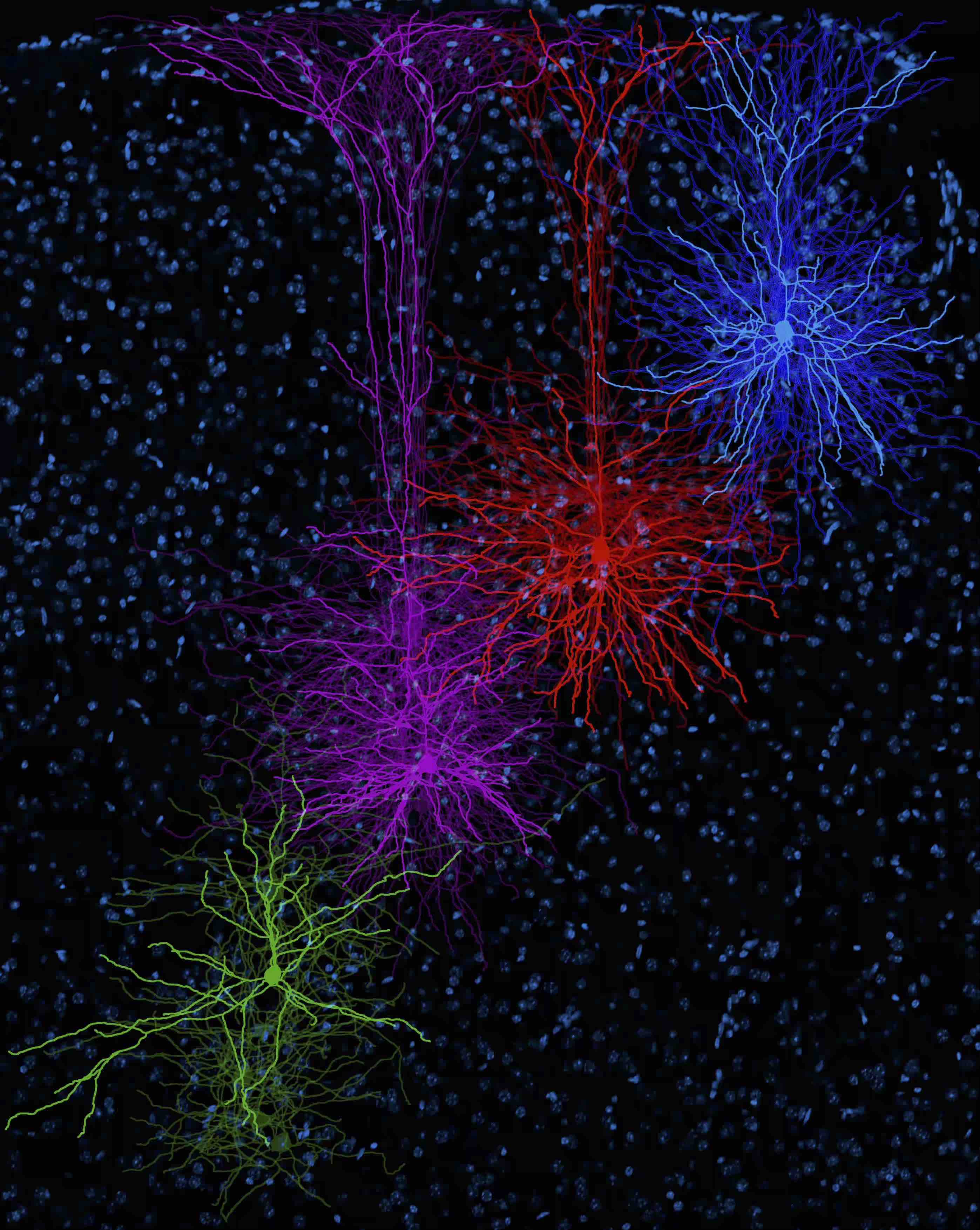
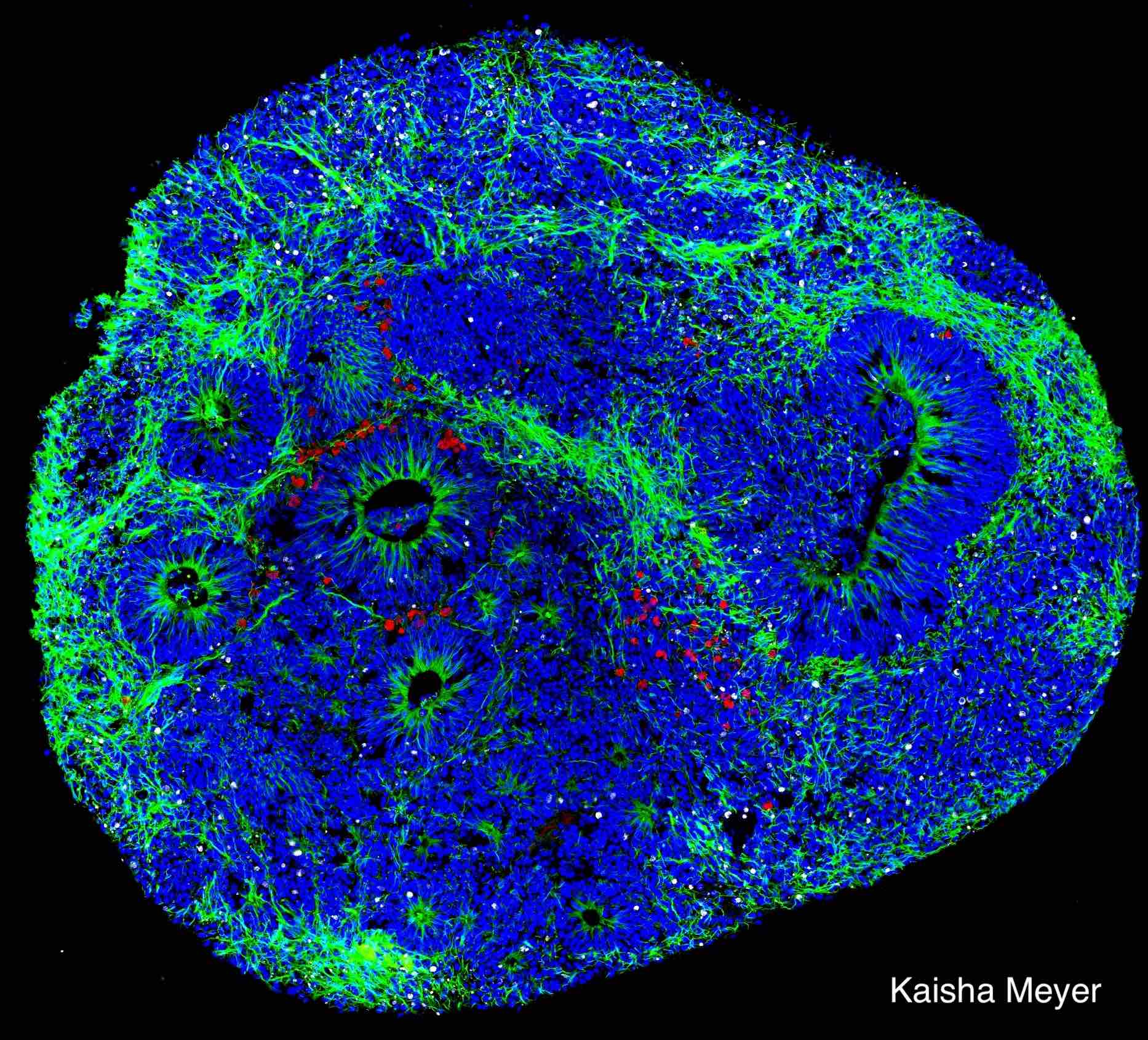
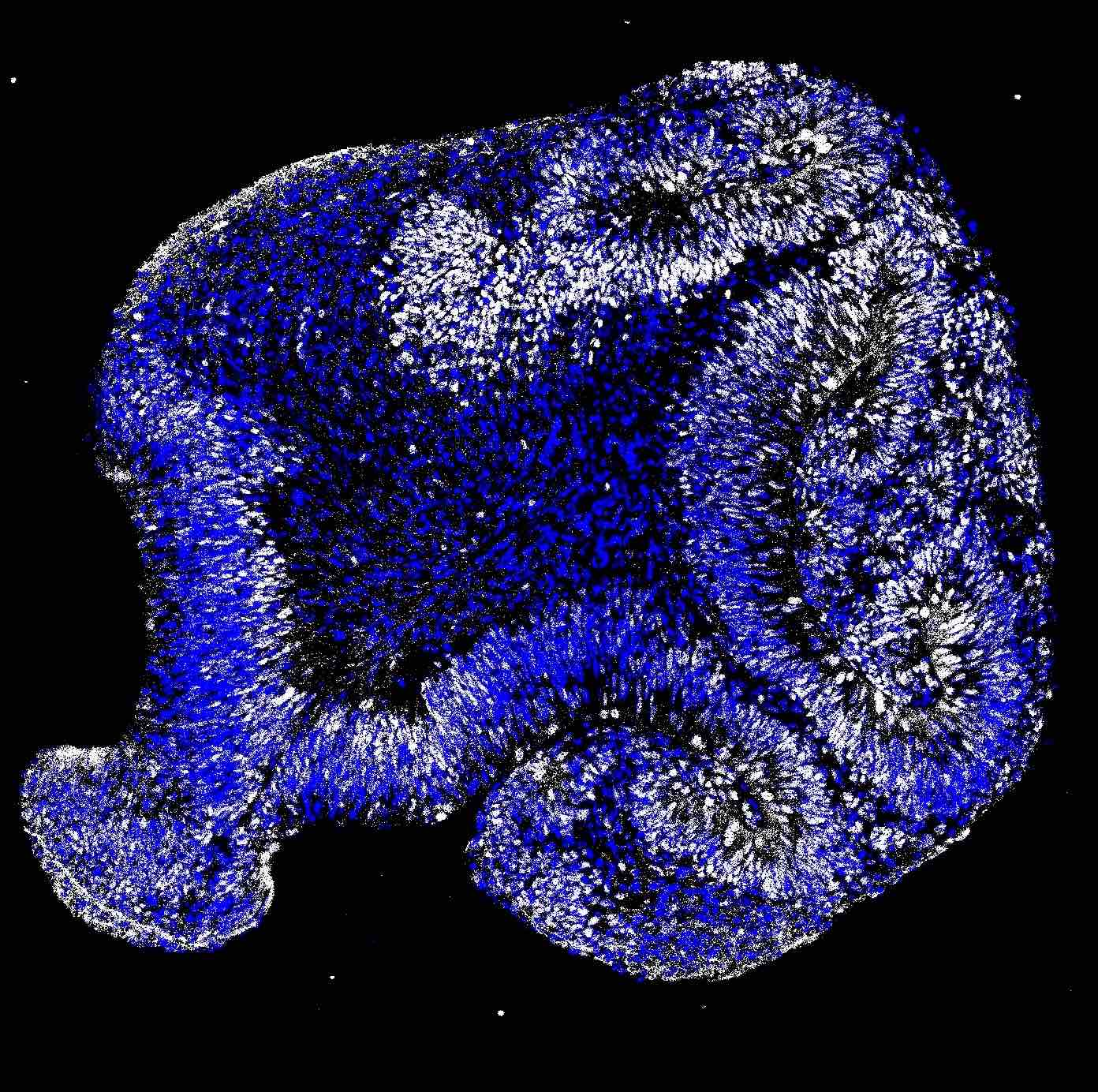
The Neuroscience PhD program at UTSA is a diverse and inclusive research community. We provide trainees with cutting-edge research opportunities and professional development to ensure that students achieve their career goals.
Neuroscience PhD Program Core Faculty
Core Faculty have labs where Neuroscience PhD students can pursue doctoral dissertation research

Alfonso Apicella, PhD
Associate Professor
The goal of Dr. Apicella's lab is to reveal the neural basis of perception. More specifically, he wants to understand exactly how cortical microcircuits process sensory information to drive behavior. To assess how populations of neurons concur to encode information, generate perceptions, and execute behavioral decisions requires working at both the cellular and system level. Towards this goal, by turning neurons "ON" and "OFF" using optogenetic and pharmacogenetic approaches, the lab can monitor and then manipulate specific subsets of neurons in awake behaving mice.

Anthony Burgos-Robles, PhD
Assistant Professor
The main interest of Dr. Burgos-Robles' lab is to understand alterations on brain function by psychological stress. Using animal models, the lab uses sophisticated tools to evaluate the evolution of stress-induced alterations in the activity of discrete neural populations and circuits. Particularly, they focus on limbic regions such as the amygdala and the medial prefrontal cortex, which are necessary for emotional learning, as well as their projections to key downstream regions that promote aversive and rewarding behavior, such as the periaqueductal gray and the nucleus accumbens, respectively.

Erika Tatiana Camacho, PhD
Professor
Research in Dr. Camacho’s laboratory focuses on mathematically modeling and investigating both the healthy and diseased retinas at the cellular and molecular levels. Her work and interest centers on 1) the metabolic needs of cones in the absence of rods, before, during and after degeneration and retinal remodeling, 2) aerobic glycolysis and oxidative stress pathways in photoreceptors and the retinal pigment epithelium, 3) metabolic pathways implicated in photoreceptor degeneration, and 4) immune response in retinal degenerative diseases. In her earliest publication in this area, her work predicted the existence of a necessary mechanism experimentally discovered a year later – the rod-derived cone viability factor (RdCVF) and proposed equations describing the dynamics of the rod and cone outer segments and the RPE cells.

Melanie Carless, PhD
Associate Professor
Dr. Carless' research focuses on identifying genetic and epigenetic factors associated with complex diseases, and in understanding how these might contribute to disease risk, and be leveraged as potential novel therapies. She is particularly interested in how epigenetic mechanisms such as DNA methylation, DNA hydroxymethylation, and microRNAs contribute to gene regulation, and consequently risk for metabolic disorders (e.g., diabetes and obesity) and neurological and psychiatric diseases (e.g., Alzheimer's disease, schizophrenia, bipolar disorder). To accomplish this, her laboratory employs a range of approaches, including cohort-based studies, post-mortem tissue analysis, animal models and cell-based systems, as well as cutting-edge technologies, including stem cells, organoids, next-generation sequencing and epigenetic editing using the CRISPR/dCas9 system.

Thomas Forsthuber, MD, PhD
Professor
Dr. Forsthuber's lab pursues several lines of investigation to understand how the immune system, in particular T cells, contribute to autoimmune diseases and how to modulate T cell immunity for therapeutic purposes in humans. Specifically, he studies immune mechanisms in the central nervous system in experimental autoimmune encephalomyelitis (EAE), the animal model for MS. Moreover, Dr. Forsthuber studies human autoimmune heart disease in a model called experimental autoimmune myocarditis. His research is aimed towards direct applicability to human diseases, for example by developing novel drugs for autoimmune diseases and biomarkers to monitor the efficacy of treatments for autoimmune diseases.

Chris Gamblin, PhD
Professor
The Gamblin laboratory studies the mechanisms that lead to the polymerization of the microtubule-associated protein tau. Tau is a protein that is important in neuronal function, but can misfold and aggregate into pathological structures that accumulate in neurodegenerative disorders such as Alzheimer's disease. The main approach of the Gamblin lab is to use a variety of small molecules to induce the aggregation of a wide array of biological variants of the tau protein to identify conditions to produce disease-relevant strains of tau in vitro for further characterization. We use these approaches to better understand the effects of modifications of tau found in disease on tau aggregation; to identify potential therapeutics to slow, stop, or reverse tau aggregation; and to identify other biological factors that may influence tau aggregation in disease.

Gary Gaufo, PhD
Associate Professor
The long-term goal of Dr. Gaufo's laboratory is to understand the mechanisms that regulate plasticity in living organisms. In its simplest definition, plasticity is the capacity of single- or multi-celled organisms to adapt to their environment. Using the mouse as a model organism, the lab's research focuses on neural crest and preimplantation embryonic cells to study plasticity. Neural crest cells are multipotent progenitor stem cells that are unique to vertebrates. In addition to giving rise to most peripheral neurons and glial cells, neural crest cells give rise to an array of cell types that make up the head. Preimplantation embryonic cells are the ultimate in vertebrate cellular plasticity; they have the potential to differentiate into both embryonic and non-embryonic tissues, which includes the placenta. These broad categories of cell types thus provide a solid platform the dissect the molecular basis of cellular plasticity in a mammalian model organism.

Ed Golob, PhD
Professor
Our lab studies aspects of hearing that are particularly important to humans, such as determining where a sound is coming from or recognizing speech and music. We examine how auditory processing is affected by attention, memory, and the relations between perception and action. We also strive to understand the cognitive and neurobiological differences that accompany normal aging, age-related neurological disorders such as Alzheimer's disease, and speech fluency disorders. In many studies we monitor the brain's electrical activity using event-related potentials and EEG while subjects perform cognitive tasks. Our lab also uses transcranial magnetic and electrical stimulation to transiently influence brain activity. Ongoing studies also employ computational modeling, AI, and MRI methods.

Joseph Houpt, PhD
Assistant Professor
Dr. Houpt's research involves mathematical psychology which is a subfield of psychology that focuses on developing and applying formal quantitative models of psychological phenomena. Most of mathematical psychology is applied to cognitive psychology, particularly memory, decision making, and reasoning.

Jenny Hsieh, PhD
Professor
The Hsieh laboratory studies the cellular and molecular markers that control neural stem cells in the hippocampus ("adult neurogenesis") as well as a "disease-in-a dish" approach, which uses patient stem cells to re-create human brain disorders in the lab. They were the first group to use a transgenic mouse to ablate adult-born granule neurons, and they showed this decreased seizure development later in life. The lab also uses optogenetic and chemogenetic tools to define the critical period and circuit mechanism that govern the aberrant properties of adult-born granule neurons in the hippocampus circuitry. To translate their work to patients, the lab uses human induced pluripotent stem cells to evaluate the role of genetic mutations in epilepsy and neurodegenerative disorders, ultimately for precision medicine. The lab's goal is to develop novel strategies to treat or prevent neurological disorders, such as acquired and genetic forms of epilepsy. or neurodegenerative disorders, such as Alzheimer's disease.

David Jaffe, PhD
Professor
Research in Dr. Jaffe's lab focuses on how individual neurons and networks of neurons process information. Their work primarily focuses on the hippocampal formation, a region of the brain important for the acquisition and consolidation of declarative information (i.e. facts and events) and one that is impacted early in Alzheimer's disease. Using a combination of electrophysiological, optogenetic, imaging, and computational approaches, the lab is currently studying how communication from the cortex is filtered through the dentate gyrus on its way into the hippocampus, and how this impacts mechanisms associated with the replay of newly encoded information during memory consolidation. The lab is also investigating how this circuit is involved in seizures and the development of epilepsy, and how seizure-induced changes in membrane ion channels (i.e. channelopathies) contribute to epileptogenesis. A second area of interest is how pain information is regulated by the dorsal root ganglion.

Hyoung-gon Lee, PhD
Associate Professor
Dr. Lee's research is focused mainly on the understanding of the pathological mechanism(s) underlying the selective neurodegeneration in Alzheimer disease and other neurodegenerative diseases. Multiple molecular mechanisms identified from previous research in the lab which would lead to the development of the effective therapy. Among these identified mechanisms, ongoing research in the lab is focused on cell cycle re-entry in neurodegeneration and insulin signaling in Alzheimer disease.

Itamar Lerner, PhD
Assistant Professor
Dr. Lerner's research focuses on the cognitive and computational neuroscience of memory and learning. He also researches the sleep effects on cognition and emotion.

Annie Lin, PhD
Associate Professor
The general theme of research in Dr. Lin's lab is cell fate regulation in the human health and diseases with focus on the intersection of stem cells and brain cancer biology. The ongoing projects seek to understand what extent stem and progenitor cells become cancer-initiating cells. Thus, the lab's work has potential implications for basic stem cell and cancer biology as well as translational significance for treatment and prevention of diseases.

Lindsey Macpherson, PhD
Assistant Professor
Dr. Macpherson’s lab investigates the connectivity and plasticity of peripheral sensory circuits, especially for taste and oral/facial somatosensation. The lab primarily uses mouse models to genetically manipulate, label, trace, and monitor the activity of taste receptor cells and peripheral sensory neurons in vivo. Specific techniques include in vivo calcium imaging, intravital 2-photon microscopy, GFP Reconstitution Across Synaptic Partners (GRASP), CRISPR knock-in/knock-out, immuno/in-situ fluorescence, RNA-seq, and behavioral analysis. Research questions include: 1) Coding: How is chemosensory/somatosensory information encoded by peripheral sensory neurons? 2) Connectivity: What are the synaptic partners of specific taste receptor cell types? 3) Dynamics: How do gustatory fibers and taste synapses change during taste cell turnover? 4) Plasticity: How do drugs, age, disease, or diet affect peripheral sensory neuron connectivity and function?

John McCarrey, PhD
Professor
Research in Dr. McCarrey's lab is centered on the development, differentiation, and epigenetic regulation of mammalian germ cells and stem cells, and on the role of the epigenome as a mediator of environmental effects. Experimental systems include mice, baboons, and other mammals. The lab is interested in 1) the potential for assisted reproductive technologies (e.g. IVF), adverse lifestyles (e.g. poor diet, lack of exercise), or environmental exposures (e.g. disruptive chemicals) to induce disease-causing epimutations in the sperm that are transmitted to a male's offspring, 2) epigenetic specification and maintenance of spermatogonial stem cell fate, 3) maintenance of enhanced genetic integrity in germline and pluripotent cells, 4) regulation of gene expression in germ cells and stem cells, 5) X-chromosome activity or inactivity in germ cells, 6) epigenetic reprogramming during gametogenesis, and 7) developing the baboon as a nonhuman primate model for studies of stem cell-based therapies.

Sandra Morissette, PhD
Professor
Dr. Morissette's research interests focus on military health, post-deployment functional recovery trauma, anxiety and addictive behaviors, and smoking cessation.

Chris Navara, PhD
Associate Professor of Research
Dr. Navara’s research focuses on the cellular biology of pluripotent stem cells. Using human pluripotent stem cells, Dr. Navara’s research group makes human nerve cells from Parkinson’s patients, tests their biology to better understand the disease, and tests new potential therapies that may slow or stop its progression. Pluripotent stem cells also hold great promise for the treatment of diseases ranging from diabetes to Parkinson’s disease. Dr. Navara’s group is using non-human primates as preclinical models for the safe testing and optimization of these therapies prior to use in the clinic.

George Perry, PhD
Professor
Dr. Perry's studies are focused on the mechanism of formation and physiological consequences of the cytopathology of Alzheimer disease. The lab has shown that oxidative damage is the initial cytopathology in Alzheimer disease. They are working to determine the sequence of events leading to neuronal oxidative damage and the source of the increased oxygen radicals. Current studies focus on the role of redox active metals in mediating prooxidant and antioxidant properties, the mechanism of phosphorylation control of oxidative damage to neurofilament proteins, and the mass spectrometry analysis of protein metal binding and crosslinking.

Gabriela Romero Uribe, PhD
Assistant Professor
Dr. Romero Uribe's research interests focus on macromolecular bio-interfaces for cell manipulation, biologically inspired drug delivery systems, soft biomedical materials, and stimuli-responsive macromolecules.

Fidel Santamaria, PhD
Professor
Dr. Sanatmaria's long term objective is to combine theory, modeling, and experiments to understand how the cerebellum computes information; develop closed-loop systems for neuronal control, particularly those associated with deep brain stimulation; and neuromorphic devices for the solution of real-time complex signal analysis for brain machine interfaces.

Francesco Savelli, PhD
Assistant Professor
Dr. Savelli is a computer scientist and AI researcher turned neuroscientist. This path inspires him to study the physical and computational nature of brain functions as a "reverse engineering" problem. One high-level function concerns the use of perceptual information of external landmarks (e.g., from the visual system) and the internal sense of motion (e.g., from the vestibular or motor systems) to dynamically create your sense of location relative to a mental map of the surrounding environment. Neurons of the hippocampal formation such as place cells, grid cells, and boundary cells appear to participate in this function. Experimental and computational work in the laboratory is motivated by several broad questions: 1) What role exactly these cells have in the computations that are necessary for creating the map and for updating your sense of location; 2) How subcortical regions participate in this process; and 3) How all this relates to other types of cognitive abstractions that the hippocampal formation creates beyond maps (e.g., of time, or of autobiographical memories).

Marina Silveira, PhD
Assistant Professor (coming January 2024)
Neuromodulators shape the organization, function, and computations of neuronal circuits. The overall goal of the Silveira Laboratory is to understand how neuromodulation impacts sound processing in the brain. In the central auditory pathway, most auditory pathways converge in the inferior colliculus (IC), which is localized in the auditory midbrain. The IC is extremely important for hearing, as damage to the IC leads to major impairments in speech comprehension and sound localization. Interestingly, the IC receives several neuromodulatory inputs, however how neuromodulators shape auditory processing in the IC and how neuromodulatory inputs to the IC change after hearing loss is largely unknown. In our lab we use in vitro and in vivo electrophysiology, optogenetics and anatomy to understand how neuromodulation impacts auditory computations in the auditory midbrain and how midbrain circuits change after hearing loss.

Alexey Soshnev, MD, PhD
Assistant Professor
Aberrant cell fate decisions due to transcriptional misregulation are central to malignant transformation and developmental disorders. Histone proteins are the major constituents of chromatin – complex of nucleic acids and proteins interpreted by cellular machinery, and mutations in histone-encoding genes are increasingly recognized as drivers of disease. Mutations in linker histone genes were recently identified as drivers of peripheral lymphoid malignancy (Yusufova, … Soshnev, Cesarman, Melnick, Nature 2021), and we aim to understand the mechanistic basis of chromatin decompaction, redistribution of core histone modifications, and reactivation of stem cell–specific transcriptional programs upon H1 loss in germinal center B-cells during malignant transformation.

Todd Troyer, PhD
Associate Professor
Research in the Troyer lab focuses on the question of how neural activity is coordinated within neural circuits to produce behavior. One set of research questions centers on studies of vocal communication in songbirds and mice. Songbirds are an excellent model system for understanding how the brain orchestrates activity on multiple timescales to produce a complex sequence of actions. A second line of research employs theoretical and modeling techniques to gain fundamental insights into how noise and variability influence computations in neural circuits.

Matt Wanat, PhD
Associate Professor
Our actions are driven by the pursuit of rewards and avoiding aversive outcomes. We are particularly interested in studying how stress and drugs of abuse influence motivation, learning, and decision-making processes. The lab employs a number experimental techniques, including fiber photometry, chemogenetics, and optogenetics. Ongoing research projects are examining the behavioral consequences of astrocyte-neuron interactions in the midbrain, the long-term consequences of stress on reward-guided behavior, and the neural circuits involved with changing reward preference. Our ultimate goal is to identify and reverse neural adaptations underlying aberrant processes in models of psychiatric disorders.

Nicole Wicha, PhD
Professor
Dr. Wicha's research focuses on understanding how the brain processes language in real time using both behavioral and brain imaging techniques. The lab has three primary areas of research that encompass their work: 1) Neural time course of language comprehension, 2) Cognitive neuroscience of bilingual language and cognition, and 3) Interface between language and other cognitive processes (e.g., math).

Charles Wilson, PhD
Professor
Dr. Wilson's lab studies the circuitry and neurons of the basal ganglia, with the goal of understanding the computational function of these structures at the cellular level, and their dysfunction in diseases, especially Parkinson's Disease. Their experiments are focused on the ionic mechanisms that endow each cell type with its characteristic responses to synaptic input, the patterns of connectivity that deliver specific inputs to each cell, and the dynamics that arise from the combination of these.
Neuroscience PhD Program Affiliate Faculty
Affiliate Faculty are not currently accepting dissertation students but can serve on dissertation committees.

Lacy Barton, PhD
Assistant Professor
Our research group explores the developmental underpinnings of fertility in early embryogenesis. We are particularly interested in a widely conserved stage when the embryonic germ cells migrate through several tissues to colonize the somatic gonad, while simultaneously undergoing many other developmental processes, including sex-specific differentiation, cell division, and population pruning. Our current focus is to understand how these processes are spatially and temporally coordinated by the surrounding soma and developing ovary and testis.

Astrid Cardona, PhD
Professor
Dr. Cardona's research is focused on understanding the mechanisms of tissue damage in Multiple Sclerosis and Diabetic retinopathy. Her laboratory focuses on the functional interactions between immune cells, microglia, neurons and blood vessels, utilizing experimental mouse models of disease, immunological assays, flow cytometry, fluorescent activated cell sorting, microscopy and molecular biology approaches.

Maria Gonzalez Porras, PhD
Assistant Professor
Dr. Gonzalez Porras’ research focuses on integrating the areas of physiology, stem cell biology, and bioengineering to develop cell targeted therapeutic systems for adipose tissue. Her research interests lie in using multidisciplinary strategies to gain a better understanding of the cellular and microenvironmental conditions fundamental to the pathogenesis and therapy of metabolic disfunctions in cancer, obesity, and diabetes.

Brian Hermann, PhD
Professor
Dr. Hermann's laboratory studies the basic biology of spermatogonial stem cells (SSCs), which are adult-tissue stem cells responsible for sperm production in the mammalian testis and which are essential for male fertility. Ongoing studies in the lab are focused on 1) how the pool of SSCs forms during development; 2) determining how SSC fate is regulated; 3) how SCC loss due to chemotherapy can be prevented; and 4) how SCCs can be used to treat male infertility. The lab's work has potential implications for basic stem cell biology, reproduction, as well as translational significance for treatment and prevention of male infertility.

Isabel Muzzio, PhD
Professor
Dr. Muzzio's research focuses on the variables that affect spatial navigation and episodic memory - events occurring in specific contexts at particular times. Her lab investigates how neurons in the hippocampus and other areas of the medial temporal lobe form representations of context that facilitate navigation and memory encoding. Specifically, she studies how these representations change when animals are lost, under conditions of stress and fear, and following sleep deprivation. Dr. Muzzio's lab addresses these questions conducting long-term single cell recordings in freely moving mice in combination with pharmacological, genetic, behavioral, and computational tools.

Robert Renthal, PhD
Professor
Research in Dr. Renthal's lab is focused on 1) Biochemistry and biophysics of cell membranes – What are the biophysical mechanisms of folding and oligomerization of membrane-embedded proteins? How do oligomeric channels form in membranes? 2) Chemical communication by insects and ticks – How do ant colonies establish and maintain interaction networks? What semiochemicals and chemoreception-related proteins are involved in mate and host identification by tick and fly vectors of human diseases? and 3) Tick immune system – Why do tick vectors harbor pathogenic bacteria, such as Lyme disease spirochetes, instead of killing or expelling them? How do Lyme disease bacteria evade the tick immune system?

Jeffrey Vedanayagam, PhD
Assistant Professor
Research in Dr. Vedanayagam’s laboratory is centered on studying the genetic renegades in the genome called selfish genetic elements and their impacts on germline development, reproduction, and fertility. In particular, our lab is interested in understanding 1) how selfish meiotic drive genes, which thwart Mendelian segregation during meiosis, compromise germline genome integrity; 2) how host suppression strategies evolve to control the activities of meiotic drive genes and restore faithful transmission of genetic information; 3) what are the consequences of genetic conflicts to the evolution of genes involved in germline processes. We primarily use Drosophila to study the molecular workings of intragenomic conflicts and also utilize computational/bioinformatics approaches to study how selfish genetic elements shape the evolution of fly and mammalian genomes. Our lab is committed to inclusivity and fosters a diverse and welcoming environment that promotes equal opportunities for learning and growth.